
·The specific oxidation mechanism of SO4- is achieved by extracting hydrogen from saturated carbon atoms and providing electrons to unsaturated carbon. It is also found that · SO4- is relatively stable in neutral and acidic aqueous solutions. When pH>8.5, · SO4- oxidizes water or OH - to form · OH (E0 is+2.8V), thus initiating a series of free radical Chain reaction.
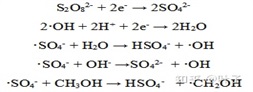
Gouttenye et al. showed that under acidic and neutral conditions (pH 2-7), the main active free radical is · SO4-, while under alkaline conditions (pH>12), it is mainly · OH. In short, under acidic and alkaline conditions, activated Sodium persulfate can produce free radicals and play its role in the oxidative degradation of organic pollutants.
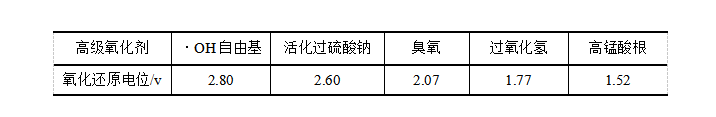
Activated Sodium persulfate is a kind of advanced oxidant. Other advanced oxidants include Fenton's reagent, Potassium permanganate and ozone. Under the reaction conditions of high temperature and pressure, electricity, sound, light irradiation, catalyst and so on, advanced oxidants can oxidize and degrade macromolecular refractory organic substances into low toxic or non-toxic small molecular substances, or even directly degrade into CO2 and H2O, nearly completely mineralized, by generating free radicals with strong oxidizing ability, addition, substitution, Electron transfer, bond breaking, etc. with organic compounds. The oxidation capacity of mainstream advanced oxidants (calculated by standard oxidation Reduction potential E0) is in the order of · OH free radical>activated Sodium persulfate>ozone>hydrogen peroxide and permanganate, as shown in Table 1.
Table 1 Comparison of oxidation potentials of major advanced oxidants
Among them, activated Sodium persulfate is a new type of oxidant in the field of environmental remediation. It has the characteristics of high stability, high water solubility (595g/L at room temperature), no odor, and strong oxidation capacity in a large pH range. Chlorinated organic pollutants suitable for treatment by activated Sodium persulfate include chlorinated alkanes, chlorinated aromatic hydrocarbons, and some chlorinated pesticides. Compared with Fenton's reagent, Potassium permanganate and other traditional oxidants, activated Sodium persulfate has less damage to Soil organic matter and indigenous microbial communities, which is conducive to the restoration of soil ecological environment functions after restoration.
In situ remediation of soil and groundwater environment, the pollutants in soil and groundwater can be removed by dissolving activated Sodium persulfate in water and injecting oxidants into the ground. Therefore, in-situ remediation generally requires the construction of several injection wells for chemicals in contaminated sites. In order to improve the mixing rate of pesticides with contaminated soil and groundwater, methods such as central injection peripheral extraction or peripheral injection central extraction can be used to accelerate the flow and circulation of pesticides in groundwater.
In recent years, more and more repair sites have adopted Geoprobe injection drill rods equipped with pressure activated drill bits to achieve pressure injection of the agents. According to the characteristics of the target soil layer, a pressure range of 0.5-5 MPa can generally be used. This injection method does not require the installation of an injection well, and can directly use Geoprobe to press the drill pipe into the target depth to inject the agent. Suitable for soil layers with low permeability such as cohesive soil and silty cohesive soil.
Due to the complexity of underground conditions, in situ chemical injection is difficult to achieve ideal uniformity in mixing with contaminated soil and groundwater compared to excavation for ectopic remediation. In many cases, even excessive injection can lead to a rebound in pollutant concentration. Therefore, in situ repair generally requires several rounds of drug injection, with each cycle typically taking one to several weeks (including the drug injection period and reaction period) until the final repair meets the standard.
In the ex situ remediation of contaminated soil excavation, Sodium persulfate is also activated as an oxidant to remove pollutants in the soil. The difference is that ex situ remediation involves excavating contaminated soil and disposing of it in situ or ex situ. Ectopic restoration areas generally require an impermeable layer (such as HDPE film or concrete) to be laid on the exposed ground to prevent secondary pollution during the restoration process. Unlike in situ repair where only liquid agents can be injected, there are three main ways to add drugs for ectopic repair:
(1) Add solid agents to contaminated soil, mix well, and then sprinkle water for maintenance. Medication is generally done through an integrated dosing and mixing device (commonly known as an 'all-in-one machine'), where both dosing and mixing are completed simultaneously. Sprinkle water until the soil is basically moist.
(2) Add dissolved agents to contaminated soil. It is necessary to first build a cofferdam around the excavated soil to prevent the loss and diffusion of chemicals. Then, high-pressure water guns and other nozzles can be used to spray chemicals on the soil surface, and excavators and other instruments can be used to stir the soil, so that the liquid chemicals are evenly distributed and exert their effects in the soil.
(3) Partially add solid agents and partially add liquid agents. Generally, solid agent particles are added first, thoroughly mixed, and then liquid agent is sprayed on the surface, followed by covering and insulation maintenance.
Another significant difference between ex situ remediation and in situ remediation is that ex situ remediation can first use quicklime to regulate the soil moisture content, especially for saturated contaminated soil located below the groundwater level. Quicklime can quickly reduce the moisture content to an appropriate level; Another main function of quicklime is as an activator of Sodium persulfate.
Generally speaking, Sodium persulfate mainly has three activation mechanisms: thermal activation, Fe2+activation, and ultraviolet light assisted Fe2+activation. Zhao Chenxi et al. used activated Sodium persulfate for remediation of petroleum hydrocarbon contaminated soil, and studied the removal efficiency of petroleum pollutants in soil under the above three activation modes. The results indicate that:
(1) The thermal/S2O82 system has a high removal rate of petroleum hydrocarbons in acidic environments. The optimal parameters are pH=2, T=50 ℃, Na2S2O8=1mol/L (equivalent to a mass concentration of 23.8%), and the reaction time is 5 days.
(2) The Fe2+/S2O82- system is more complex under the influence of pH, and strong acidic, neutral, or alkaline environments are not conducive to the degradation of organic pollutants. Excessive addition of Fe2+or Sodium persulfate will make SO42- in the system react with each other, trigger free radical quenching, and reduce the removal rate of pollutants. The optimal parameters are pH=2, T=50 ℃, Na2S2O8=1mol/L, Fe2+=1mol/L, and the reaction time is 5 days.
(3) Under the synergistic conditions of ultraviolet light and Fe2+, the degradation rate of petroleum hydrocarbons in soil can be improved. The optimal repair parameters for the UV/Fe2+/S2O82 system are pH=4, Na2S2O8=1mol/L, Fe2+=1mol/L, and UV lamp irradiation for 4 days. Among them, the interaction between pH/Fe2+concentration and Fe2+/S2O82- concentration can significantly affect the removal rate of petroleum hydrocarbons.
(4) Under the three activation methods, pollutants in the soil are all removed. Among them, the synergistic activation method of ultraviolet light and Fe2+has the highest removal rate, followed by the Fe2+activation method, and the thermal activation method has the relatively lowest removal rate.
As mentioned earlier, when pH>8.5, some · SO4 can oxidize water or OH - to generate · OH -; At pH>12, most free radicals are · OH -. Due to the slightly higher oxidation ability of · OH - than · SO4-, utilizing the · SO4- and · OH - active radicals generated by activated persulfates under alkaline conditions can accelerate the oxidative degradation of pollutants.
The commonly used activation method of quicklime (CaO) in practice combines thermal activation and alkali activation to form the alkali thermal activation method. On the one hand, the activation of Sodium persulfate is realized through the heat release of lime when it meets water, and on the other hand, the alkaline condition formed when lime is dissolved in water. Due to the convenience and low cost of lime extraction, this method is widely used in practice.
Activated Sodium persulfate advanced oxidation method is increasingly widely used in soil and groundwater remediation, which has good effects on petroleum hydrocarbons, volatile organic compounds, and some semi volatile organic compounds. The main problems currently prevalent are excessive dosing and secondary pollution.
(1) Excessive dosing: According to practical experience, the problem of excessive dosing is common when Sodium persulfate is used for environmental remediation. The reasons for excessive dosing are, on the one hand, to ensure that the restoration meets the standard, and on the other hand, the geological and hydrogeological conditions of the site are constantly changing, making it difficult to achieve uniform distribution of the agent in the soil and groundwater in one dosing, or to achieve the desired restoration effect, often requiring multiple repeated dosing; During this process, it is easy to encounter the problem of excessive dosing. Once again, the failure to conduct small-scale to medium-sized trials in the early stages of the repair, or inaccurate experiments, may also lead to excessive dosing.
(2) Secondary pollution: whether in situ or ex situ remediation, the degradation process of Sodium persulfate after oxidation produces a large amount of sulfate, acidity or alkalinity (when alkali is activated), causing salinization or acidification of soil and groundwater. Thus causing damage to the utilization value of the repaired site. For example, low pH may cause harmful heavy metals in soil to dissolve into groundwater, increasing the likelihood of migration. For example, the salinization of soil limits its potential for greening purposes.
Activated Sodium persulfate advanced oxidation technology is increasingly applied to the treatment and remediation of volatile and semi volatile organic pollutants such as petroleum hydrocarbons and polycyclic aromatic hydrocarbons. Generally speaking, this technology ensures the repair effect and the repair cycle is relatively short. The activation modes of Sodium persulfate mainly include heat, light (UV), transition metal ions (Fe2+, Ag+, Ce2+, Co2+, etc.), as well as alkali activation and alkali heat activation. According to theoretical analysis and practical experience, alkali thermal activation with quicklime may be the best Sodium persulfate activation method at present.
Founded in 2012 and listed on the NEEQ on May 20, 2016, Shanghai Huanzuan Environmental Protection Technology Co., Ltd. is an early Environmental engineering company engaged in the core business of contaminated site investigation, risk assessment and restoration project implementation in China. Environmental drilling has rich construction experience in advanced oxidation remediation of organic pollution sites.
Reprinted from: Polaris Environmental Restoration Network News